By continuing to use this site, you agree to our use of cookies as described in our Cookie Policy.
| Multiple myeloma | Mantle cell lymphoma | |
|---|---|---|
| Previously untreated disease |
|
|
| Relapsed or refractory disease |
|
|
| Pre-existing or at high risk of peripheral neuropathy | ||
| Hepatic impairment |
|
|
Please refer to the full Prescribing Information for direction regarding administration and dose modifications.

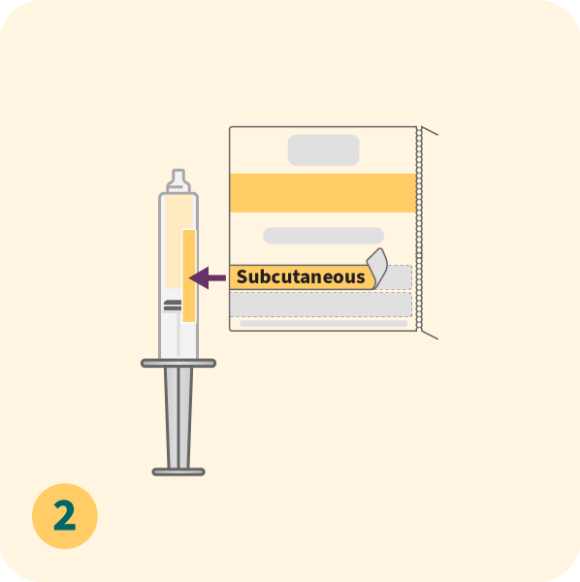
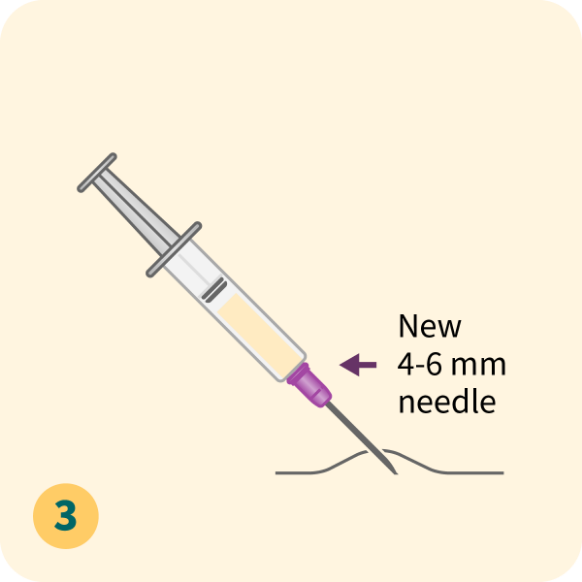
Please refer to the full Prescribing Information for dose modification and treatment cycle recommendations.
Rotate the sites for each injection
(thigh or abdomen).1

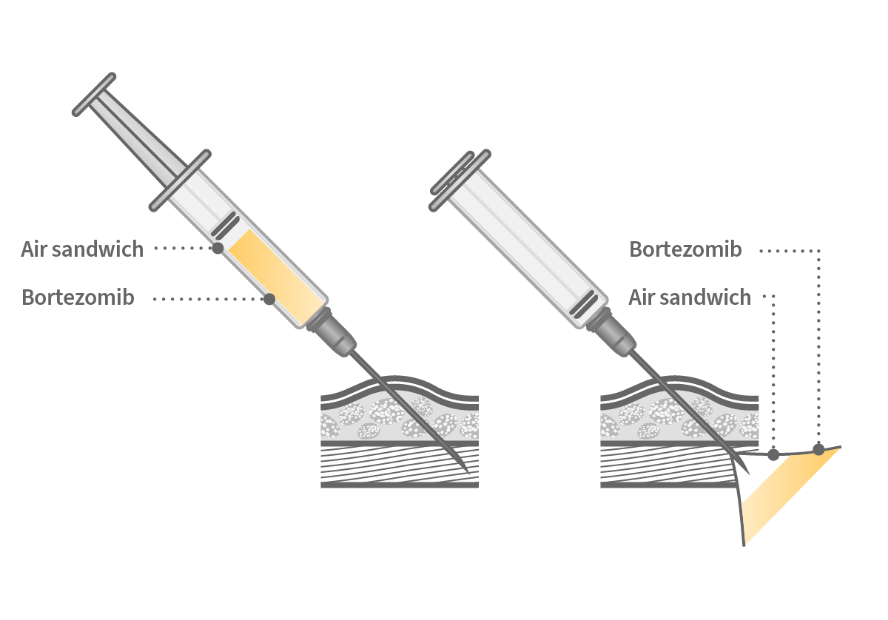
Consider using the air sandwich (also known
as air lock) injection technique.4-6
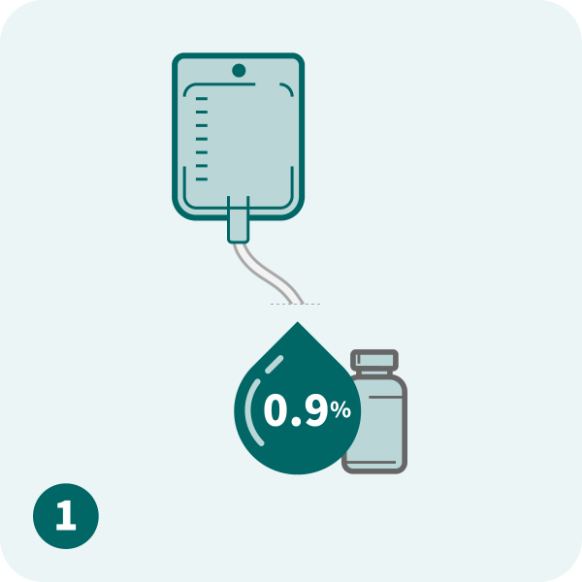
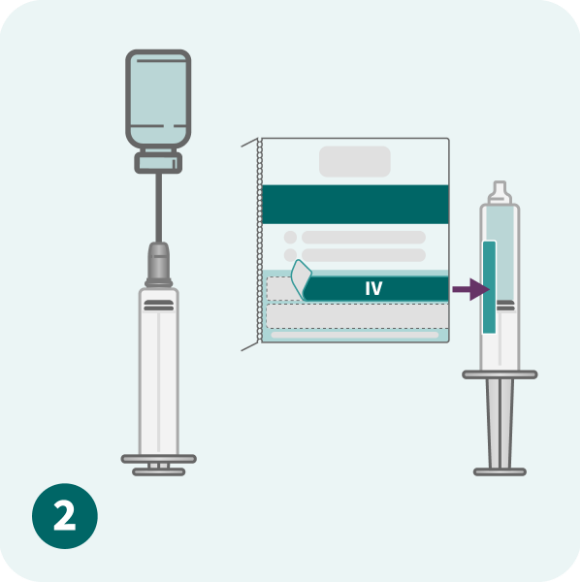
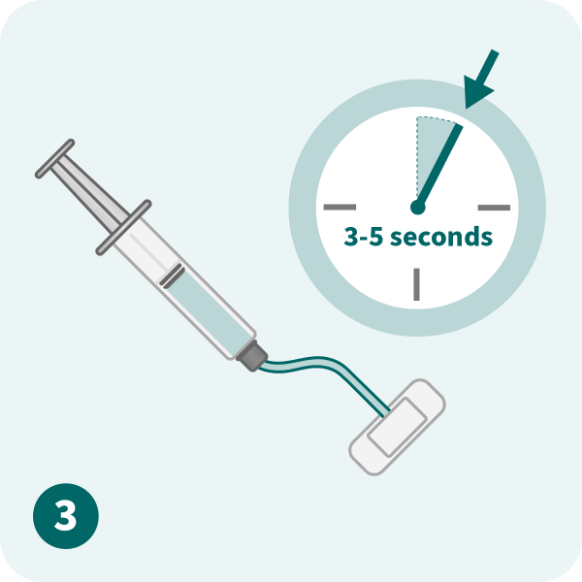
Please refer to the full Prescribing Information for dose modification and treatment cycle recommendations.
Use caution when calculating the volume to be administered.
NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
*While it is possible to administer BORUZU® intravenously for patients who have or are at risk for peripheral neuropathy, subcutaneous administration is preferred.1,3
BSA, body surface area; IV, intravenous.
References: 1. BORUZU. Prescribing information. Amneal Pharmaceuticals LLC; 2024. 2. Arnulf B, Pylypenko H, Grosicki S, et al. Updated survival analysis of a randomized phase III study of subcutaneous versus intravenous bortezomib in patients with relapsed multiple myeloma. Haematologica. 2012;97(12):1925-1928. 3. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Multiple Myeloma V.1.2025. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed February 4, 2025. To view the most recent and complete version of the guideline, go online to NCCN.org. 4. Kurtin S, Knop CS, Milliron T. Subcutaneous administration of bortezomib: strategies to reduce injection site reactions. J Adv Pract Oncol. 2012;3(6):406-410. 5. Boudreau A. Practical considerations for the integration of subcutaneous targeted therapies into the oncology clinic. Can Oncol Nurs J. 2019;29(4):267-270. 6. Becze E. Make subcutaneous administration more comfortable for your patients. January 4, 2022. Accessed January 6, 2024. https://www.ons.org/publications-research/voice/news-views/01-2022/make-subcutaneous-administration-more-comfortable
BORUZU® is a proteasome inhibitor indicated for the treatment of:
BORUZU® is for subcutaneous (SC) or intravenous (IV) administration only. Because each route of administration has a different final concentration, caution should be used when calculating the volume to be administered.
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Biosciences, a division of Amneal Pharmaceuticals LLC at 1-877-835-5472 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.